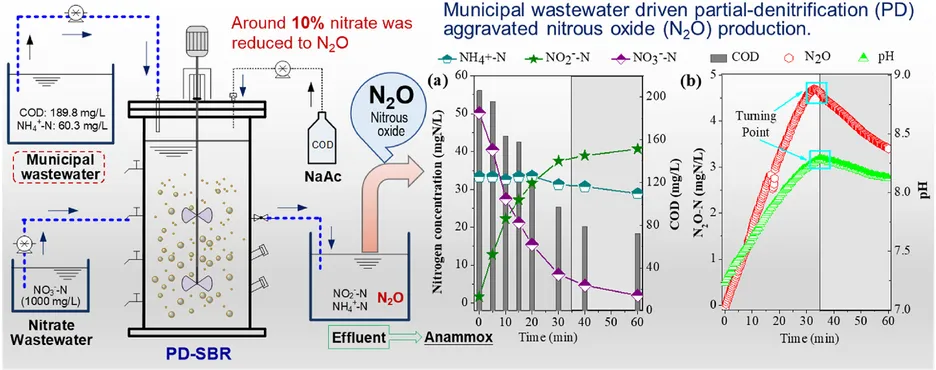
Partial Denitrification (PD) provides a new route for nitrate-rich wastewater treatment by combining with energy-efficient anammox. This process was reported to hardly produce N2O despite high nitrite accumulation using acetate as carbon source. This study, however, demonstrated significant N2O would be produced when feeding with real municipal wastewater, the NTR-N2O (NO3−-to-N2O transformation ratio) reached as high as 10%. The supply of feed with a high COD/NO3−-N ratio was unable to alleviate N2O production but was severely aggravated after nitrate was depleted. Excess carbon source resulted in accumulated nitrite further being reduced with NTR-N2O largely improved to 63.4%. Besides, it was observed that acid pH inhibited PD activity and resulted in N2O production significantly increased with the NTR-N2O of 39.9% at an initial pH of 5.5. The increase of pH to alkaline conditions cannot mitigate N2O production. A low feeding proportion of real municipal wastewater for low nitrate wastewater treatment can drastically lower N2O production. The NTR-N2O was only 0.1% at a nitrate concentration of 10 mgN/L (10%), much lower than that at 30 mgN/L with a 30% feeding proportion of municipal wastewater (9.4%). Complex organics and ammonium present in municipal wastewater were supposed to be the primary reasons for the high N2O production. Overall, this work offered a new insight into N2O production during the PD process using the complex organics from wastewater itself.