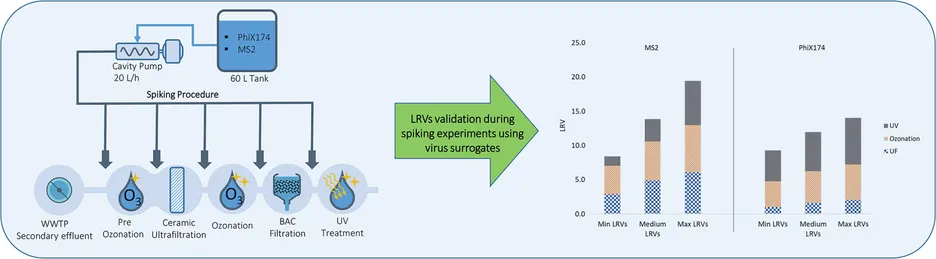
In this study, we evaluated the ability of various pilot-scale treatment train combinations to meet the microbial requirements of the new European non-potable water reuse regulation 2020/741. The study utilized non-disinfected secondary effluent from the wastewater treatment plant in Schweinfurt, Germany, as feedwater for two pilot-scale treatment trains. The first, a reference treatment train (Train A), consisted of filtration and UV disinfection as specified for reclaimed water class A in the EU regulation. The second, an advanced treatment train (Train B), included ceramic ultrafiltration (UF), ozonation, biological activated carbon filtration (BAC), and final UV disinfection. Based on a Monte Carlo simulation for Train A, the EU requirements for pathogen removal were not met when an average UV dose of 400-600 J m−2 was applied. This shortcoming was likely due to a moderate transmittance range (50–65 %), resulting in decreased UV fluence. These findings suggest that operational conditions for disinfection should be more clearly specified to ensure consistent pathogen inactivation both during validation and regular operation. In contrast, treatment train B successfully met the requirements of the EU regulations by reducing pathogens to below the detection limit. The UF membrane demonstrated a positive effect on the overall log reduction values (LRVs) throughout the water reclamation system. It also enhanced the efficiency of downstream processes, such as ozonation and UV disinfection, by lowering total suspended solids and turbidity. However, even without the UF membrane, treatment train B was still able to meet the pathogenic EU requirements for non-potable reuse applications. Furthermore, the study observed that the inclusion of biologically activated carbon (BAC) filtration requires a final disinfection step (e.g., UV disinfection) to prevent the potential occurrence of heterotrophic bacteria that proliferate in the BAC filter. For process validation it is recommended to use at least two different virus surrogates (MS2 and PhiX174), rather than just one or total coliphage as required in the EU regulation.