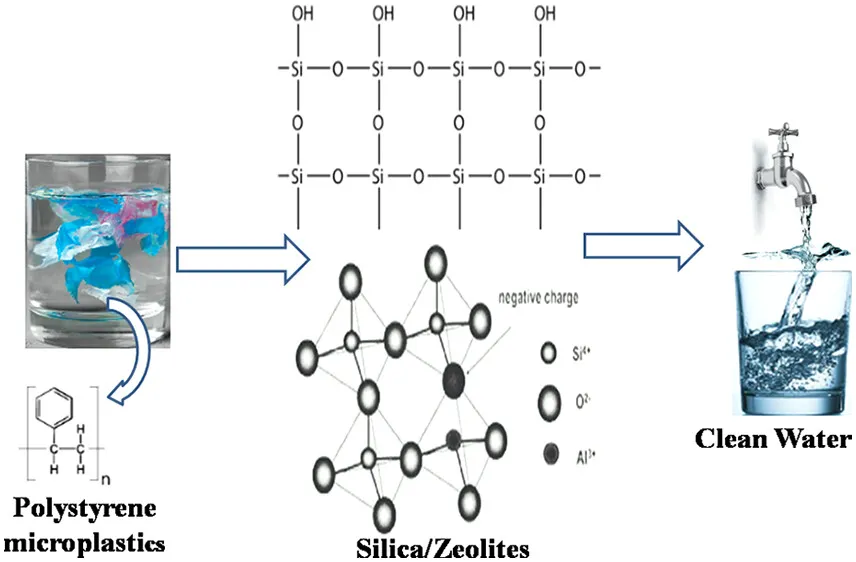
Recently, the challenge of environmental microplastics (enMPs) in ecosystems has become a serious global concern. This is because the transport of enMPs has been known as a precarious culprit in depleting ecosystems, likely decreasing life expectancy, reducing the quality of human life, and threatening the future survival of fauna and flora. This menace is seriously threatening the continued existence and well-being of all biomes. Hence, this research attempts to provide a panacea to this global environmental issue through the application of Santa Barbara Amorphous silicas/zeolite composite (SSZC) for the removal of polystyrene microplastics (PMPs) from water and wastewater. This research showed that the adsorption capacity of SSZC for PMPs was 2.41 mg·g–1. This was achieved by chemisorption between SSZC and PMPs via electrostatic attraction and hydrophobic interactions, such as covalent bonding, noncovalent aromatic π-system, and electron donor–acceptor interactions. The surface morphology of SSZC showed that C–H, C–O, C═C, N–H, Al–O, Si–O–Si, and Si–OH were the functional moieties present on its surface and available for adsorption.